
Targeting China’s economic aggression
In the White House Diplomatic Reception Room yesterday, President Trump made it clear he’s taking a stand for American innovation.
“We’ve lost, over a fairly short period of time, 60,000 factories in our country—closed, shuttered, gone. Six million jobs, at least, gone. And now they’re starting to come back,” the President said. “But we have one particular problem. And I view them as a friend . . . and that’s China.”
The President has directed his Administration to consider a range of actions to respond to China’s unfair and harmful acquisition of U.S. technology. Now, the Administration is proposing for public comment adding 25 percent additional tariffs on products that are supported by China’s unfair industrial policy.
“The word that I want to use is ‘reciprocal,’” President Trump said. “When they charge 25 percent for a car to go in, and we charge 2 percent for their car to come into the United States, that’s not good.”
Watch: President Trump signs a Memorandum targeting China’s economic aggression
Learn more: How we must level the playing field for American workers
What advice would you give?
Americans born between 1982 and 2000 represent more than a quarter of our Nation’s population. These millennials now outnumber baby boomers, and they’re becoming a growing force in our country’s policy debates.
A booming economy, more jobs, and free speech protections—especially on America’s college campuses—are just some of the ways the Trump Administration is helping millennials. Yesterday, the White House hosted about 150 of these young men and women for panel discussions and an interview with President Trump.
Charlie Kirk, founder of the conservative nonprofit Turning Point USA, asked the President questions about taxes, trade policy, and free speech. But the question that sparked the most reaction? “What advice would you give to the 25-year-old Donald Trump, knowing what you know today?”
Watch Charlie Kirk’s full interview with President Trump here.
PHOTO OF THE DAY

President Donald J. Trump and Archbishop Demetrios at the Greek Independence Day celebration | March 22, 2018 (Official White House Photo by Joyce N. Boghosian)
POTUS TODAY
This morning, President Trump and Vice President Pence will meet with the Secretary of Defense, the Secretary of Homeland Security, and the Deputy Secretary of State.
MedWatch - The FDA Safety Information and Adverse Event Reporting Program
BD Vacutainer Blood Collection Tubes by Becton, Dickinson and Company (BD): Class I Recall - Chemical Interference with Certain Tests
BD Vacutainer EDTA Lavender, Tan, and Pink Top Tubes
BD Vacutainer Lithium Heparin Green Top Tube
AUDIENCE: Risk Manager, Nursing, Laboratory
ISSUE: BD is recalling their Vacutainer EDTA Blood Collection Tubes with lavender, tan, pink and green rubber tube stoppers due to a chemical in the rubber tube stopper that interferes with the accuracy of the Anodic Stripping Voltammetry (ASV) testing methodology. ASV is the methodology used in Magellan Diagnostics’ LeadCare Testing Systems. The tube stoppers contain a substance called thiuram that can sometimes release sulfur-containing gases, which may dissolve into the blood sample and bind the lead particles. This chemical reaction makes it difficult for the Magellan lead tests to detect the correct amount of lead in the sample and may cause falsely lower test results. Falsely lower test results may lead to improper patient management and treatment for lead exposure or poisoning. The use of affected product may cause serious adverse health consequences.
The tubes can continue to be used with other non-ASV blood lead level test technologies such as Graphite furnace atomic absorption spectroscopy (GFAAS) and Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and other assays (non-lead) which do not use ASV methodology.
BACKGROUND: BD Vacutainer EDTA Blood Collection Tubes are used to collect blood samples from a vein (venous). The tubes are used to transport and process the blood samples for testing in clinical laboratories.
RECOMMENDATION: On March 22, 2017, BD sent an Urgent Medical Device Correction Notice to all affected customers and distributors. The notice asked them to:
Continue to follow FDA’s safety communication for testing lead levels, which currently recommends that no venous blood should be tested with Magellan’s LeadCare test systems (regardless of the collection tube used).
Determine the need to evaluate tests performed in your facility for the potential of thiuram interference.
At FDA’s request, BD conducted testing to determine whether clinical laboratory tests other than the Magellan lead tests are affected by this interference. Based on the results of BD’s study, the FDA has no reason to believe other tests are impacted by this issue. BD provided additional data for tubes with thiuram versus tubes without thiuram. See the full list of Chemistry and Immunoassays Tests in the Recall Notice.
In addition, BD continues to investigate the potential of thiuram interference with additional tests used in clinical laboratories. Additional tests include metals, cardiac markers, cancer markers, therapeutic drug monitoring tests, and toxicology tests. However, laboratories should determine the need to evaluate their tests for the potential of thiuram interference if a test is not included in the list.
Share the notification with other relevant personnel.
Complete the Customer Response Form and return to the BD contact noted on the form to acknowledge receipt of the notification.
Customers or distributors with questions may contact BD at 1-888-237-2762 (select Option #3 and then Option #4) between 8AM and 5 PM CT Monday through Friday.Healthcare professionals and patients are encouraged to report adverse events or side effects related to the use of these products to the FDA's MedWatch Safety Information and Adverse Event Reporting Program:
Complete and submit the report Online: www.fda.gov/MedWatch/report
Download form or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178Read the MedWatch Safety Alert, including a link to the recall notice, at:
https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm602480.htm
| ||||||
|



 The Federal Trade Commission has filed a complaint and motion for preliminary injunction in federal district court alleging that Alliance Security Inc., a home security installation company, and its founder, directly and through its authorized telemarketers, called millions of consumers whose numbers are on the National Do Not Call (DNC) Registry. Two of Alliance’s authorized telemarketers and their principals also have agreed to settle charges that they made illegal calls on Alliance’s behalf.
The Federal Trade Commission has filed a complaint and motion for preliminary injunction in federal district court alleging that Alliance Security Inc., a home security installation company, and its founder, directly and through its authorized telemarketers, called millions of consumers whose numbers are on the National Do Not Call (DNC) Registry. Two of Alliance’s authorized telemarketers and their principals also have agreed to settle charges that they made illegal calls on Alliance’s behalf.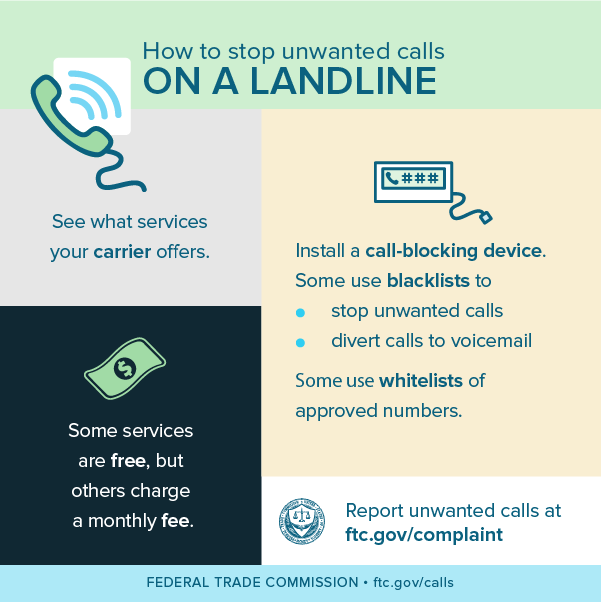 The complaint also charges Defend America and Power Marketing with violating the TSR by not identifying the seller in their calls, and Alliance for telling the two companies not to identify it in calls to consumers. The complaint also alleges Alliance and Power Marketing deceived consumers by misrepresenting themselves as calling on behalf of ADT, an unrelated home security company.
The complaint also charges Defend America and Power Marketing with violating the TSR by not identifying the seller in their calls, and Alliance for telling the two companies not to identify it in calls to consumers. The complaint also alleges Alliance and Power Marketing deceived consumers by misrepresenting themselves as calling on behalf of ADT, an unrelated home security company.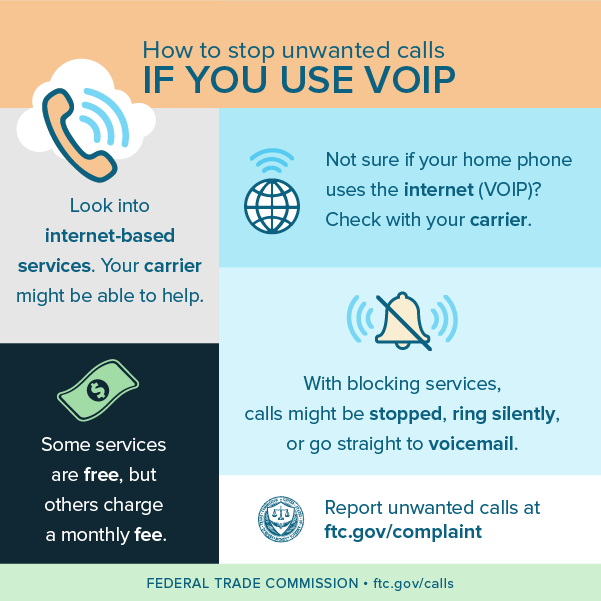 The proposed court settlements announced today resolve the FTC’s charges against individual defendants Merrick and Klink, and corporate defendants Defend America LLC and Power Marketing Promotions LLC. Litigation continues against Jasjit “Jay” Gotra and Alliance Security Inc., formerly known as Versatile Marketing Solutions, Inc.; VMS Alarms; VMS; Alliance Security; Alliance Home Protection; and AH Protection.
The proposed court settlements announced today resolve the FTC’s charges against individual defendants Merrick and Klink, and corporate defendants Defend America LLC and Power Marketing Promotions LLC. Litigation continues against Jasjit “Jay” Gotra and Alliance Security Inc., formerly known as Versatile Marketing Solutions, Inc.; VMS Alarms; VMS; Alliance Security; Alliance Home Protection; and AH Protection.
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου